- DZL
- Research
- DZL Academy
- News
- Service
International research team reveals key mechanisms of scarring in Long-COVID and validates new progression biomarkers.
(Aachen, Hannover, Mainz, Wuppertal, 05 October 2022) More than two years after the start of the global COVID-19 pandemic, the causes of the long-term consequences of SARS-CoV-2 infection, described as Long-COVID syndrome, are still not fully understood. In particular, the long-term changes in lung tissue following severe COVID-19 disease pose significant limitations for many patients. Some of these patients continue to develop so-called post-COVID pulmonary fibrosis, which is characterised by rapid scarring of the lung tissue. Until now, to the dismay of many of those affected, there has been a lack of a profound understanding of the underlying mechanisms of this scarring and of specific blood markers that can predict this scarring process. Now, an international research team led by Prof. Dr. Danny D. Jonigk, Institute of Pathology at the RWTH Aachen University Hospital and Professor of Pathology at the Hannover Medical School (MHH) and at the DZL BREATH site in Hannover, PD Dr. Maximilian Ackermann from the Institute of Pathology and Molecular Pathology at the HELIOS University Hospital in Wuppertal and from the Institute of Anatomy at the University Medical Center Mainz, and Univ. Prof. Dr. Dr. Detlef Schuppan, Institute for Translational Immunology at the University Medical Center Mainz, have used a holistic research approach to uncover the previously unknown mechanism that contributes significantly to the connective tissue remodelling of the lung in severe COVID-19. The study has now been published in the renowned journal "The Lancet - eBioMedicine".
The term pulmonary fibrosis covers a variety of different interstitial lung diseases in which persistent inflammation leads to progressive scarring of the lung scaffold. Although these serious diseases can be mitigated somewhat with medication, they are still incurable and usually have higher mortality rate than many cancers. For many of those affected, lung transplantation is the only remaining, life-saving therapy. All forms of pulmonary fibrosis are preceded by chronic inflammatory damage to the lung tissue. Epidemiological data from patients with severe COVID-19 suggest that approximately 20% of hospitalised patients develop post-COVID pulmonary fibrosis, which varies greatly in its extent and progression and can only be predicted very imprecisely by routine clinical imaging.
To reveal the microscopic tissue alterations in this scarring process in detail for the first time, the researchers of the international team examined the lungs of severely diseased COVID-19 patients using the novel technology of Hierarchical Phase Contrast Tomography (HiP-CT), the most brilliant synchrotron radiation device in the world, at the European Synchrotron Research Facility in Grenoble, France. With this tool, they were able to show for the first time that in severe COVID-19 trajectories, there is a mosaic-like change in the lobuli, the smallest lobules of the lungs, and a reduced nutrient and oxygen supply of the tissue due to changes in the blood vessels supplying the lungs. In the course of intensive care treatment, there was also an increasing extent of new blood vessel formation via a specific mechanism, so-called intussusceptive angiogenesis. This blood vessel formation, which is characteristic of COVID-19, increased significantly over the course of the COVID-19 disease and was followed by connective tissue remodelling of the lung tissue, in particular of the vessels in the lobular septa, a phenomenon which the team led by the experienced thoracic pathologist Prof. Jonigk had already observed in other forms of pulmonary fibrosis. "The formation of new blood vessels is a characteristic of the progression of fibrosis in many forms of interstitial lung disease. It is precisely the many newly formed blood vessels, which can arise via intussusceptive angiogenesis that supply the tissue with more oxygen in the short term, but also with inflammatory cells, which intensify the process all the more," Jonigk summarises the mechanisms that contribute to the increase of scar tissue leading to fibrosis. "These heterogeneously distributed, distinct changes at the level of the finest lung lobules cannot be detected at all via clinical imaging due to the lack of detail of current clinical technology. Thus, with the new technology of HiP-CT, we were able to show for the first time that the scarring processes in post-COVID fibrosis are the result of generalised vascular damage caused by the SARS-CoV-2 virus."
The researchers have also analysed tissue samples using nanostring and MALDI-TOF technology. These innovative high-throughput methods permit to identify molecular markers based on proteomics and metabolomics data, thus literally finding the "needle in the haystack". Using both technologies for the first time in severe COVID-19 and other chronic lung diseases allowed the reseachers to identify new tissue markers of severe COVID-19 and interstitial pulmonary disease. "We see a significant increase in inflammatory markers and markers of new blood vessel formation as appear to be particularly responsible for the clinical deterioration of affected patients over the course of COVID-19 disease," says Dr. Jan-Christopher Kamp from the Hanover-based Lung Research Group, summarising the extensive molecular data. "It is precisely the correlation of the molecular data with the morphological pattern of damage that is crucial." For this purpose, the research team has for the first time compared tissue from patients who died of severe COVID-19 diseases "morpho-molecularly" with tissue samples from the most common interstitial lung diseases. In doing so, they were able to identify partial overlaps with the common patterns of damage in pulmonary fibrosis, such as the so-called "NSIP and UIP patterns", but COVID-19 demonstrated molecular patterns that were specific to severe COVID-19 disease. "Our goal was to shed light on the severe COVID-19 courses that precisely promote or cause the development of pulmonary fibrosis. Even though the consequences of long-COVID are the subject of broad public discourse, we see a clear scarring pattern of damage in long-COVID, which shows many parallelisms with severe interstitial lung diseases," Ackermann adds to the comments of his colleagues from Aachen and Hanover.
It was particularly important for the international research team not only to investigate tissue markers of severe COVID-19 disease, but also to measure them in the blood serum of patients with mild and severe COVID-19. They were able to identify three "key markers", which can be summarised as "matricellular biomarkers". The levels of these matricellular proteins increase early in the blood when the progressive fibrosis process of the lung tissue begins, but when this process is still reversible and thus treatable. The scientists also found highly elevated blood levels in patients with other forms of pulmonary fibrosis, such as idiopathic pulmonary fibrosis (IPF), as well as in patients with acute respiratory distress syndrome (ARDS), which were somewhat but insufficiently reduced by state-of-the art drug therapy. "For us, the first comprehensive comparison of blood and tissue biomarkers in COVID-19 and IPF patients provided the opportunity to correlate early morphological and molecular features of the development of pulmonary fibrosis with increased levels of the involved proteins in the blood," said Univ. Prof. Dr. Dr. Detlef Schuppan from the University Medical Center Mainz and Harvard Medical School, summarising the comprehensive results of the study. "Clinically, we have long lacked reliable predictive blood markers that help us identify pulmonary fibrosis patients and to predict the progression of fibrosis. These so-called 'predictive serum biomarkers' enable us to find new therapeutic approaches and to start therapy for the affected patients as early as possible, when the scarring processes can still be reversed via drug therapy, and also to predict the success of different therapies at an early or later stage," Schuppan sums up the great benefit of the new matricellular (macrophage, vascular) biomarkers for patients with long-COVID or pulmonary fibrosis.
The work is part of an international research network of Aachen, Antwerp, Basel, Boston, Grenoble, Heidelberg, Copenhagen, Mainz, Port St. Lucie, Munich and Wuppertal and was supported by renowned research institutions, including the European Research Council (ERC), the US NIH, the German DFG, the Chan Zuckerberg Initiative CZI and the UK Wellcome Trust.
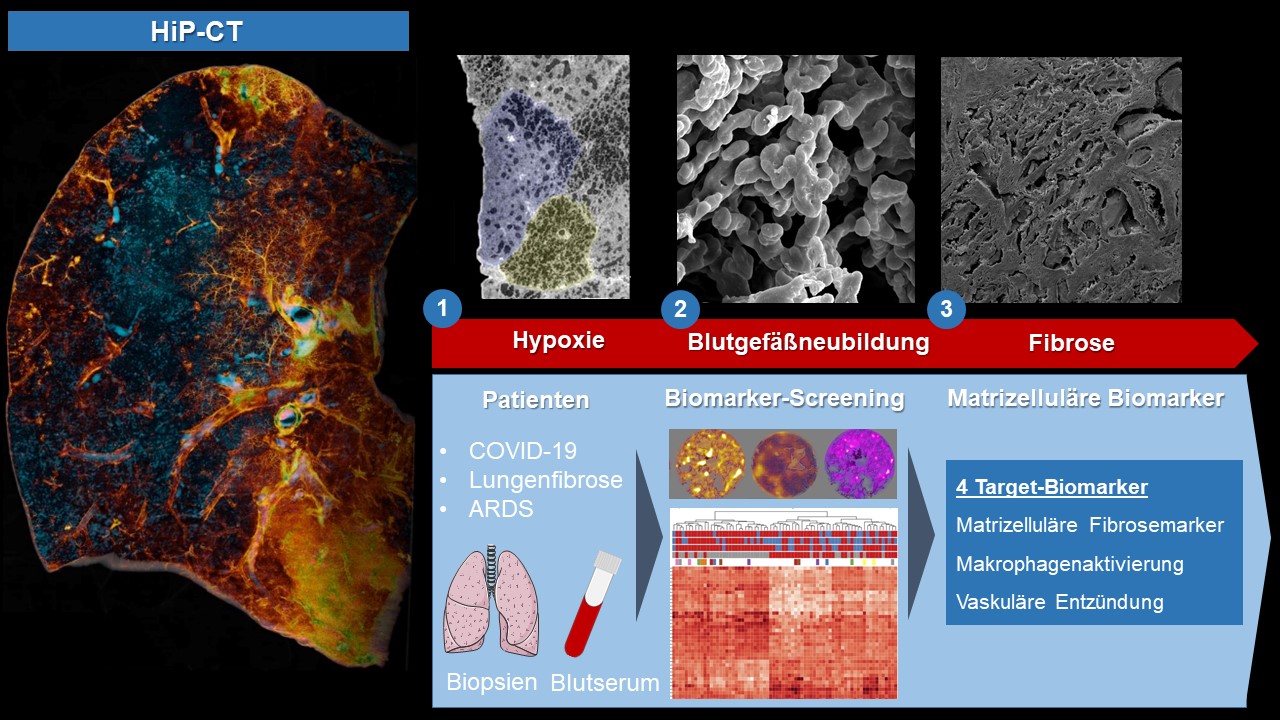
Figure. With the help of synchrotron-based hierarchical phase-contrast tomography (HiP-CT), a mosaic-like hypoxic undersupply of the smallest functional unit of the in severe COVID-19 lungs, the lung lobules, could be shown for the first time. This hypoxia and vascular damage caused by the SARS-CoV-2 virus leads to excessive formation of new blood vessels, so-called intussusceptive angiogenesis, which leads to scarring and fibrosis of the lung tissue in a very short time via inflammatory processes. In order to identify potential therapeutic targets or progression biomarkers, blood serum and biopsy tissue from patients with different COVID-19 progressions, pulmonary fibrosis (IPF) and acute lung injury (ARDS) were analysed and validated in a broad screening approach using proteomics and metabolomics. Three matricellular biomarkers and one macrophage-derived biomarker were identified as predictive blood-biomarkers that predict the progression of the scarring process.
Image source: Paul Tafforeau, ESRF; Claire Walsh, UCL; Maximilian Ackermann, Universitätsmedizin Mainz
Ackermann M*, Kamp JC*, Werlein C, Walsh CL, Stark H, Prade V, Surabattula R, Wagner WL, Disney C, Bodey AJ, Illig T, Leeming DJ, Karsdal MA, Tzankov A, Boor P, Kuehnel MP, Laenger FP, Verleden SE, Kvasnicka HM, Kreipe HH, Haverich A, Black SM, Walch A, Tafforeau P, Lee PD, Hoeper MM, Welte T, Seeliger B, David S, Schuppan D*, Mentzer SJ*, Jonigk DD*. Lobular ischemia and fibrotic remodelling drive the fatal trajectory of pulmonary COVID-19. The Lancet - eBioMedicine. Volume 85, 2022 Oct; 04; https://doi.org/10.1016/j.ebiom.2022.104296; * shared first or senior authorship
Walsh CL, Tafforeau P, Wagner WL, Jafree DJ, Bellier A, Werlein C, Kühnel MP, Boller E, Walker-Samuel S, Robertus JL, Long DA, Jacob J, Marussi S, Brown E, Holroyd N, Jonigk DD, Ackermann M, Lee PD. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat Methods. 2021 Dec;18(12):1532-1541. doi: 10.1038/s41592-021-01317-x.
- Univ.-Prof. Dr. Danny D. Jonigk, FRCPath, Director of the Institute of Pathology, University Hospital of RWTH Aachen, phone.: +49 241 80-89280, E-Mail: djonigk@ukaachen.de
- PD Dr. Maximilian Ackermann, Institute of Pathology and Molecular Pathology, Helios University Hospital Wuppertal, University of Witten-Herdecke, and Institute of Anatomy, University Medical Center Mainz, phone.: +49 202 896-2460, e-mail: maximilian.ackermann@uni-mainz.de
- Univ.-Prof. Dr. Dr. Detlef Schuppan, Director of the Institute for Translational Immunology, University Medical Centre Mainz, phone.: +49 6131 17-7356, e-mail: detlef.schuppan@unimedizin-mainz.de